


|
| About Marshall Future Students Current Students Alumni Faculty/Staff |



|
|
|
|||||
|
PS.122 Links:
» PS.122 home - - - Unit 1 - - - » topic 1 » topic 2 » topic 3 - - - Unit 2 - - - » topic 4 » topic 5 » topic 6 - - - Unit 3 - - - » topic 7 here! ⇒ » topic 8 » topic 9 - Off-Campus Sites -
» physicsforums
|
Physics For Teachers (PS.122 - §102, 2019 Fall => CRN 3671)
Class Meets in : Science 179 ... Tue & thRs 6:30pm - 8:20pm My office: Science 159 (below ramp to 3rd Ave) e-mail : foltzc @ marshall.edu phone : (304) 696-2519 Quiz 7 was
| |||||||
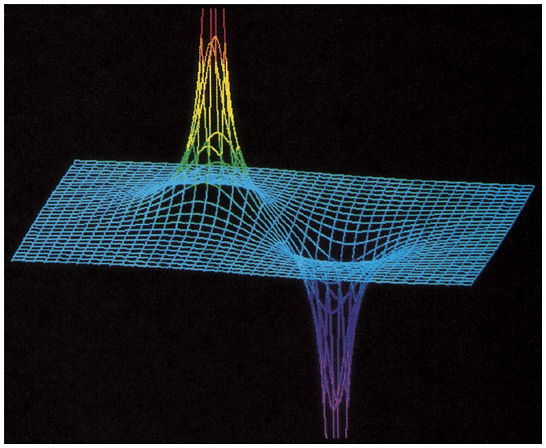 | here's a 2-dimensional graph of the Electric Potential (vertical axis)
near a positive charge (left) and a negative charge (right) everywhere on the left half has positive Potential V = 0 all along center-line , midway between the charges
|
Power : the rate that charge carries Energy
A fresh battery has a lot of (+) PE because it has excess protons (+q) near the (+)V end,
and also a lot of excess electrons (−q) near the (−)V end.
. . . in use, electrons leave the (−)V end with (−e)(−V) = (+)PE,
and eventually enter the (+) end with (−e)(+V) = (−)PE .
It is the difference in potential (one end − the other) that is the voltage, which describes the "electrical landscape".
the Electric Field is the gradient (slope, distance-wise) of the potential , so that's what determines how fast the charge flows.
. . . connect a really long wire to both sides of a battery, and the potential gradient along it will be very shallow, so very slow current
connect that drop via a short route, and the current might be so fast as to melt its insulation off!
if there are 2 routes from high PE to low PE, some charge will take each route ... more will take the steeper or wider wire
. . . the current that enters the split is the same total current after the split − it is just split
the shorter filament wire with big Area conducts well , so it presents low resistance to charge flow
=> most of the current takes the path of least resistance
. . . if there are 2 obstacles (light bulbs) in one path (from high to low), the 2 voltages ("electric height" drops) must add to be the total voltage
current will flow ½ as fast (I = ½ I1) because each bulb (filament length) can only be half as steep ... since their voltages add.
=> I = V /R . . . where R is the Resistance to flow ... "obstacle-ness" ... Resistances in series add ... since the same current flows thru both bulbs!
sort-of-optional: more about electrical resistance
You may think of resistance as electric friction, transforming electric PE into Thermal TE
the faster the current goes thru, the faster the Energy gets "lost to friction" ... since Power = ΔE/Δt ,
=> Power = I·V . . . Watt = Amp·Volt .
resistance depends on the length of the conductor × how hard that kind of material is to go thru
. . . conductance ... 1/resistance ... depends on the Area that the electrons can go thru
=> R = resistivity * L / A . . . resistivity is a material property (look up in a table) but it changes with Temperature
Resistances in series add ... because their lengths add
2 resistors in series exert 2× the resistance (to current flow) as one did
. . . resistances in parallel are reduced ... conductances in parallel add ... because their Areas add
3 resistors in parallel exert 1/3 as much resistance as one did
| maintained by Curt Foltz - email comments to foltzc@marshall.edu
... all my pages are copywrite as "Fair Use" (name me as source) my pages don't use cookies, or collect any info from your browser but read this Privacy Policy for info on "www.marshall.edu" pages. | Marshall University
One John Marshall Drive Huntington, WV 25755 (304) 696-3170 | A-Z MU site index
MU Academics MU Calendars financial aid |