Topic 10 Summary :
Translational Kinetic Energy of the atoms (which constitute an object) is interpreted as Temperature .
Each atom's motion is in a random direction at any instant, so the total momentum of the object is zero
. . . relative to the center-of-mass , of course ... if the object as a whole is moving, pobject = Σ pi , all i atoms
Each atom stays in the object, so the average momentum of each atom is zero (relative to the c.o.m.) .
. . . but the KE is never negative , so the average KE is not zero .
The total Mechanical Energy (relative to c.o.m.) in the atoms which compose the object is called its Thermal Energy
abbreviated T.E. (or Internal Energy , I.E)
. . . on average, I.E. is partitioned equally among all the modes which can contain Energy :
=> E = ½ kB T , in each mode , for each atom . . . kB = 1.38E−23 [Joule/Kelvin] = 86¼ [micro·eV/Kelvin]
With 1[kg] at room Temperature having N ≈ 5E25 atoms at T ≈ 290[Kelvin] , T.E. ≈ 100,000 [J/kg] in each mode.
If Energy is deposited into an object , randomly , the Temperature will increase :
ΔE = N (# modes) ½ kB ΔT = C ΔT . . . this capital C is called the object's Heat Capacity .
. . . the specific Heat Capacity ... lower case c ... essentially divides out the Number of atoms in the object.
In a sample of monatomic gas , for example , each molecule has 3 modes of motion which can contain Energy :
. . . ±px , ±py , ±pz ...
=> TEtotal = KEtotal = 3/2 N kB T , for a collection of N monatomic molecules .
Diatomic molecules , and polyatomic molecules , also contain Energy in rotational motion ,
. . . around any axis that can have non-zero angular momentum
an axis thru the c.o.m. can have angular momentum only if some mass is non-zero distance from that axis . . .
. . . straight molecules (along x-axis, say) have 2 rotational modes : ±Ly , ±Lz
=> TEtotal = 5/2 N kB T , for N straight molecules . . .
. . . bent molecules hold Energy in rotations around the x-axis : ±Lx also
=> TEtotal = 6/2 N kB T , for N bent molecules .
at high Temperature , other motions in the molecule are also excited (flexing , atoms vibrating in their PE well)
(once the Action ~ KE·Δt = KE / f in those modes are large enough ( > 6¾ E−34 [J·s] )
Condensed matter (solid and liquid) also contain Potential Energy , which is always negative ,
but becomes less negative ... higher ... when Energy is added.
. . . the PE function for real condensed matter is not easy to calculate , so their specific heat capacities
are carefully measured , and tabulated for different materials ... usually listed for T ≈ 273[K] or 300[K] .
. . . caution : c is slightly higher at higher Temperatures , for most materials .
Only the translational KE ; from ±px , ±py , ±pz , influences a thermometer .
These Energies are randomly distributed among atoms , and among motions , and among times
. . . sometimes, an atom will have more E than average , at other times it will have less .
some atoms have more E than average , at any instant (others have less then)
The likelihood of some atom having its px equal to some value is proportional to exp [−E/kT] = exp [−p²/2mkT]
. . . the likelihood of an atom having KE between Eo and Eo + dE is the same decreasing exponential . . .
But since speed = √(vx² + vy² + vz²) , regardless of direction , velocity interval dvx·dvy·dvz = v² cos φ dθ dφ dv .
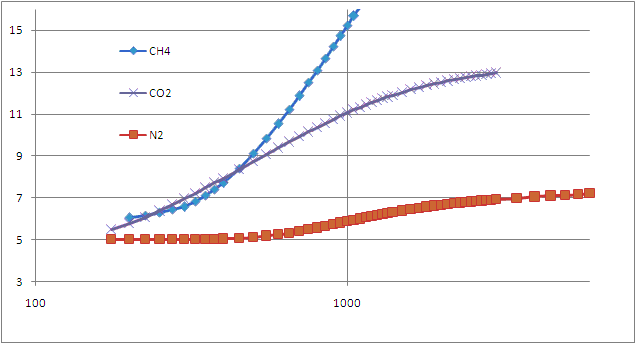
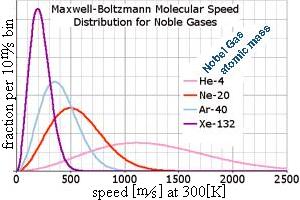
=> the fraction of atoms with some speed goes as f(v) = (A) v² exp [−½mv²/kT] ,
. . . where (A) is needed so all the fractions add up to 100% ... integrate speed (0 → ∞) to find A = √(²/π)·√(m/kT)³
 . . .
. . . 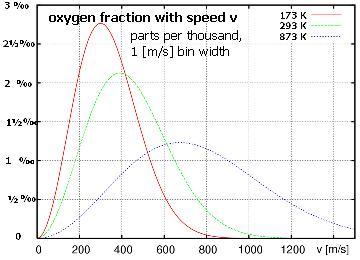
. . . the "peak" of this function is the most likely speed , occurs at √(2kT/m) . . .
. . . the average speed is a bit faster , at √(8kT/πm) since there's no such thing as negative speed . . .
. . . but the average KE corresponds to the average v2 , at √(3kT/m) because there's a long tail of fast speeds .
Thermal Expansion of Condensed Matter
Atoms in solids and liquids are trapped in the PE well caused by their neighboring atoms
. . . we can model this by the Morse Potential : PE(r) = U(r) ≈ εo { exp[−2a(r − Ro)] − 2 exp[−a(r − Ro)] } .
here , a and εo depend on the kinds of atoms involved .
. . . it's easy to see that U(Ro) = εo . . . you should be able to verify that the radial Force Fr = 0 , at r = Ro ,
and evaluate the kurvature at Ro , to see that Ro is a stable equilibrium , with spring constant k = 2a²εo .
. . . if an atom in this PE function has Energy (−εo + ε) ... that is , just ε more than the minimum PE ,
then its turning points are at : rturn = Ro + ¹/a * ln[1 ± √(ε/ε0) ]
=> rturn ≈ Ro + ¹/a [ ±√(ε/εo) + ½ (ε/εo) ± ¹/3 (ε/εo)^3/2 + ... ]
ε = ½ kB Troom ≈ .012 [eV] , while typical atoms have εo ≈ 2 [eV] , and ¹/a ≈ ½Ro .
. . . so rturn − Ro ≈ ± 0.027 Ro + 0.0008 Ro .
. . . naively we expect atom-to-atom distance to increase by 1.0008 × , with 300[K] Temperature increase .
=> 3 parts per million for each 1 Kelvin rise in Temperature .
But the Force acting at the distant turning point is weaker than the Force acting at the close turning point
so that the atom spends more time turning around at the distant turning point .
. . . so , while the average ε = ½ kB T , easily ... the time-averaged distance is not trivial to calculate .
=> raverage = Ro + α T ; look up α in a table , as a property of the material
. . . caution : α does vary slightly with Temperature , especially for complicated molecules .
Since the 0 Temperature length of an object is Lx = Nx Ro ,
=> ΔL / Li = α Δ T , measured from its length at the initial Temperature
A liquid sample has arbitrary Length , but does have measurable Volume ; V = Lx × Ly × Lz
=> ΔV / Vi = 3 α Δ T = β Δ T , measured from its Volume at the initial Temperature
Quantity of Thermal Energy Transfer : Heat Q
Heat Capacity : Thermal Energy to raise an object's Temperature by 1[K]
. . . larger Temperature change will take that many times as much Energy
=> Q = C ΔT , capital C is the (total) Heat Capacity for the object .
. . . the more atoms the object has , the more Energy it will require .
"number of atoms" is often represented by number of moles, or "mass of atoms" or Volume of atoms" , etc . WATCH UNITS !
=> Q = "m" c ΔT , where lower case c is the specific heat capacity for the material . see UNITS in your table !
Latent Heat Capacity : Thermal Energy to change the configuration of the atoms ("phase transition")
. . . without changing the Temperature . . .
While melting, rotational modes of motion must be given ½kT of Energy , because molecules in solids cannot rotate
. . . and some Energy goes into the molecule-to-molecule PE , so they can rotate as a liquid .
=> Q = m Lfusion (Internal Energy leaves as mass freezes)
While evaporating, molecules must be completely freed from their neighbor's PE well
. . . and end up with the right number of motion modes (as a gas) that have Energy.
=> Q = m Lvaporization (Internal Energy leaves as mass condenses)
In other phase transitions, solids change from one crystal structure to another
. . . usually requires Potential Energy to increase the average atom spacing .
=> Q = m Lphase change
rate of Thermal Energy Transfer : Power 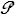 = Q / Δt
= Q / Δt
Conduction of Thermal Energy through (Condensed) Matter
Thermal Energy diffuses from Energetic (hot) atoms , to less Energetic (cold) atoms , during collisions .
even as each atom stays essentially in the same place . . . thermally agitated but near its PE minimum .
. . . larger cross-section Area that the Energy can flow through, the more will flow .
. . . larger Energy differences lead to larger Quantity transfered in each collision , on average .
Temperature gradient = δT / δx ignores sizes of the atoms
=> 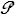 = k A dT/dx ; look up Thermal Conductivity k , a material property , in a table . . . k is NOT kB !
= k A dT/dx ; look up Thermal Conductivity k , a material property , in a table . . . k is NOT kB !
Resistance to Thermal Energy Flow :
especially for building insulation materials , where various thicknesses can be obtained , dx / k = R is used.
. . . often called the "R-value" of that insulation thickness , it is usually in old Imperial Units
=> 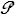 = A δT / R ; the R-value of multiple layers on the same Area is simply Reffective = Σ R .
= A δT / R ; the R-value of multiple layers on the same Area is simply Reffective = Σ R .
Convection : Thermal Energy Moves WITH the (fluid) Matter
If a hot object is carried into a cold environment , the hot object brings Thermal Energy with it.
. . . if the hot object stays in the cold environment very long, some Thermal Energy will transfer to that environment.
blowing hot air into a cold room by a fan, or pumping hot water into a cold room , are Forced Convection .
Natural Convection : if hotter below
Hotter fluid has lower density than colder fluid of the same type ,
. . . the more different the densities , the faster the hotter fluid will rise , as the cold fluid sinks
δP = δρ g h . . . goes as ½ρ v² , so v ~ √(2gh δρ/&rho) ~ √(2gh β δT) .. . . ignoring viscosity !
. . . the more Thermal Energy in the hot matter , the more Energy is transfered : 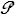 = (dm/dt) c δT = ρ v A c δT
= (dm/dt) c δT = ρ v A c δT
=> 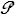 ≈ ρ c A √(2gh β) δT^3/2
≈ ρ c A √(2gh β) δT^3/2
Thermal Radiation (ElectroMagnetic) :
Electric charges shake off Electromagnetic Radiation = "light" , while being accelerated.
. . . if the charge oscillates with higher frequency , its acceleration Aω² is more violent ,
so it emits more energetic light waves (higher frequency light waves have more E , and look a different color)
=> the most Power is emitted as light with E = 4.965 kB T = 0.43 [milli·eV/K] T .
. . . with wavelength 2900[μm K] / T , since light speed = 3E8 [m/s] .
Room Temperature 300[K] emits most 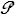 at λ =9.67[μm] light with E =129[meV] , appears as InfraRed .
at λ =9.67[μm] light with E =129[meV] , appears as InfraRed .
Solar surface Temperature 5800[K] emits most 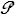 at λ =0.50[μm] light with E =2.5[eV] , appears yellow-green .
at λ =0.50[μm] light with E =2.5[eV] , appears yellow-green .
The bigger the surface Area , the more Energy can go thru it ;
. . . the hotter the surface , the more varied is each acceleration ;
. . . the more energetic the charge , more energy it can radiate in the various acceleration values ;
. . . the faster the charge , the more often these accelerations occur ;
=> 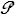 = σ ε A T 4 ; here , σ = 5.67E−8 [W/m² K 4] , and ε is surface emissivity : 1 for soot, ½ for clay, ¼ for Al
= σ ε A T 4 ; here , σ = 5.67E−8 [W/m² K 4] , and ε is surface emissivity : 1 for soot, ½ for clay, ¼ for Al
room Temp (300[K]) black wood-stove walls emit 459[W/m²] ... hot stove walls (600[K]) emit 7350[W/m²] !
. . . emissivity depends (greatly) on wave frequency , so its average varies (somewhat) with Temperature .
air is transparent to visible light, so emissivity = absorptivity = 0 for most of the Sunlight Energy
. . . but is opaque to InfraRed light => Earth's IR heats the bottom layer of air ("greenhouse effect")
(to