|
 |
Phy.211
Links :
» topic 1
» topic 2
» topic 3
» topic 4
» topic 5
» topic 6
» topic 7
» topic 8
» topic 9
» topic 10
» topic 11
(here !)
» MU-online
(Web HW)
» physicsforums
(HW.help)
» 211 Syllabus
|
 |
 |
 |
Intro Physics I (Phy.211) 2010 Fall
Unit 4 ; Topic Eleven - Thermal Gas Cycles
Plan for Unit 4 :
-Topic 10:
Temperature & Thermal Energy : Ch.19 § 2,3,4 ;
IE in condensed matter : Ch.20 § 2,1,4,3 , Ch.37 §1
. . . (HW due Thr.Dec.03)
Quiz Fri.Dec.04
-Topic 11:
Ideal Gas & Work by P ; Ch.19 §1 , Ch.20 §5,6 , Ch.
Engines & Refrigerators ; Entropy : Ch.21 § 1,2,3,4
. . . (HW due Wed.Dec.09)
- plan for Exam 4 Tue.Dec.14, 10:15
Home-Work PRACTICE QUESTIONS for Topic 11 ...( NOT for grading ...):
(numbers refer to Ohanian Physics for Engineers,3rd ed.)
Ideal Gas Pressure : ch.19 prob. 9,10,12,14,15,16,17,18,19,22,23,27,28,31,32,33,35,36,38,39,40
P dV Work : ch.20 prob. 70,71,73,74,75,76,77,79,80,81
Adiabatic process : ch.20 prob. 82,83,84,85,87,88 ; Ch.21 prob. 4,5(Q=0),
ΔIE , Q , W : ch.21 prob. isobar: 7,8,9,10,11 ; isochor: 14 ; isotherm: 15,17,18 ; 1,3,12,19,43,45,46
cycles : ch.21 prob. 21,23,25(Thi/Tlo?),27 ; 29,33,35,42 ; 39,41,51
Entropy : ch.21 prob. 53,54,55,56,57,59,62,64,65,68,71,73,75
Topic 11 Homework Questions will be graded by mu-online (WebCT)
logon using your "901" number (not "name") ... and "PIN" number (not "password") ... go to "assessments"
Topic 1 Reminders : (to topic 1 summary)
USE UNITS all thru your answer ... don't just append them onto the final number !
Put symbols on a sketch, as you read . . . use meaningful phrases : "v_match" , "catch" , "ground" "turn-around"
. . . show process spans as brackets or vector arrows , with words like "average" , "losing" , "gaining" , "t=0→2" ...
Write statements as symbols first ... each statement has a SUBJECT ... manipulate symbols before plugging numbers.
. . . keep track of adjectives such as "initial" , "average" , "final" , "stopped" . . . "change"
Topic 2 Reminders : (to topic 2 summary)
subscripts for "total" or "system" , "part A" ...
keep track of the SOURCE for the external Forces applied to the (passive) object
Recognize whether you are predicting based on theory . . . reasoning from causes to their effects ... (what should it do?)
or whether you are deducing from observation . . . (what does this imply about it?)
Get un-stuck (often) by wondering "why isn't it the same as it used to be?"
Topic 3 Reminders : (to topic 3 summary)
it's the Sum of external Forces that cause an object's mass to accelerate subscripts for "total" or "system" , "part A" ...
once you separate vectors into components, keep each component separate from the others!
ΣF = ma , for each component separately.
Topic 4 Reminders : (to topic 4 summary)
Name each Force by the source of that Force ... its cause ... a function of the environment.
spring Force : Fspring = − k s ... opposite the stretch vector .
Pressure Force : Fspring = P A generally ... in a fluid , δP = ρ g δh .
Gravity : every mass in the universe contributes to the gravity field g at any place of interest.
... add "nearby" contributions as vectors ... gby M = G M / r²M-to-point (toward M) . . . then use Fgravity, on m = mg ; NOTICE : m ≠ M !
Topic 5 Reminders : (to topic 5 summary)
Energy is conserved quantity : Σ Ebefore + Σ Wby F's not in PE list = Σ Eafter .
KE = ½ m v² = ½ p·v = ½ p²/m , in any of these equivalent forms .
Each Force either does Work during motion , or has an associated PE function ... not both .
=> Wby F = F·Δs .
=> − dU / dr = Fr , the component along that direction (r)
PEgravity,local = m g h , with h measured upward (z-direction) from a "zero-height reference" point
PEelastic = ½ k s² . . . stretch s must be measured from its relaxed length
PEPressure = P·V ; it is okay to measure from a non-zero reference pressure , but be consistent !
PEGravitation = − m GM / r .
There IS NO PE for Friction Force ... must be explicitly included as a "Work by Forces not in the PE list"
Topic 6 Reminders : (to topic 6 summary)
Some Energy forms are somewhat complicated to calculate, at a fundamental level, such as Chemical PE or Nuclear PE.
. . . they can be treated as experimentally-measured quantities which are "released" (transformed) during some conversion process (i.e, burning).
more material holds more Energy ; it is the PE density (PE/Volume) or the specific PE (PE/mass) that is measured.
efficiency is the fraction (or %) of the input Energy that is transformed into the "desired form".
. . . because total Energy is consserved, the sum of efficiencies in all output forms must be 100%.
Power is the rate that Energy is transformed (or transfered) into some other type (or to some other object)
=> P = ΔE / Δt , reported in [Watt] = [J/s] .
Because KE goes as v² = vx² + vy² + vz² , motion along each component adds in an independent manner .
. . . in particular, radial and angular motion components can be treated separately for objects in orbit
=> L = r × p is conserved then , so that KEangular = L²/2mr² is a useful formula .
Topic 7 Reminders : (to topic 7 summary)
just as in straight-line motion, a thing's angular momentum tends to stay the same.
obtain angular motion formulas and equations by directly replacing each linear quantity by its angular sibling
. . . angular location, or angular position, angular orientation φ [radians] = (arc length) s/r <=> x, y, or z .
. . . angular velocity ω [radian/sec] = dφ/dt = v/r is analogous to linear velocity
. . . angular acceleration α [radian/s²] = dω/dt = a/r is analogous to linear acceleration .
. . . angular inertia I [kg m2] = Σ m r² is analogous to mass
=> Angular momentum L = r × p ... rite rist along r, pingers point to p , thumb shows L can write as L = I ω
r × F = Torque τ . . . right rist along r , fingers flip thru angle φ till point along F ; Thumb points ( | ) along Torque
. . . Torque is the angular analogy to (linear) Force => Σ τ = dL/dt . . . sometimes useful to write as Σ τ = I α
Work (a scalar) is its own analogy ... Wrot = τ ·Δθ . . . Power P = τ · ω
Kinetic Energy is its own analogy ... KErot = ½ I ω² = ½ L ω = L²/2I
Topic 8 Reminders : (to topic 8 summary)
massive objects often oscillate around their equilibrium location with natural frequency f = 1/T
... Energy will be (approximately) conserved , so KE will be maximum at the PE minimum , the equilibrium location.
... if PE = ½ k x² , the Time for the oscillation will be the same regardless of Amplitude
=> the oscillation angular frequency ωosc. [rad/sec] = √(k/m) .
x(t) = A cos (ω t + φ) . . . <=> . . . x(t) = A cos (2 π t/T + φ) ;
v(t) = − A ω sin (ω t + φ) . . . <=> . . . v(t) = − A (2 π/T) sin (2 π t/T + φ) ;
a(t) = − A ω² cos (ω t + φ) . . . <=> . . . a(t) = − A (2 π/T)² cos (2 π t/T + φ) .
... Pendulums are gravity-driven oscillators, so the gravitational mass (in the PE) cancels the inertial mass (in ω's denominator)
the PE's "k" decreases as cos(θ) , so large angle amplitudes (>30°) take longer to oscillate.
=> small-angle pendulums oscillate with ωosc. ≈ √( (dτ/dθ) / I ) .
. . . mass-on-string "simple" pendulum has ωosc. ≈ √(g/l) .
Topic 9 Reminders : (to topic 9 summary)
waves carry KE and PE (equal amounts of each) from the source (emmitter) to the receiver (absorber)
. . . PE depends on square of Amplitude that the material is displaced from its equilibrium ;
KE depends on square of maximum velocity that the material has (when crossing equilibrium).
. . . wave speed depends on the properties of the material (medium) thru which it travels
=> vwave-material = √F·l/m = √F/(m/l) ... Interaction Force divided by linear mass density m/l .
Sources that repeat with a well-defined frequency produce waves with a well-defined length in the medium :
=> vwave-relative-to-source = λmaterial fsource . . .
. . . Waves with a well-defined wave-length are encountered by a receiver with a well-defined frequency :
=> vwave-relative-to-detector = λmaterial fdetect . . .
Energy in a wave is often nearly constant as it travels , but it usually becomes more spread out
wave Intensity is Power per unit Area ; as the wave passes thru larger Area , I decreases (~1/r² if 3-d medium)
Topic 10 Reminders : (to topic 9 summary)
Hot objects contain more Energy than cold objects : E = N (# modes) ½ kB T , in each mode , for each atom . . . kB = 1.38E−23 [Joule/Kelvin] = 86¼ [micro·eV/Kelvin]
. . . only 3 modes (px, py, pz) are perceived by thermometers as Temperature ; other modes can also store Energy
Heat Q transfers Thermal Energy from hot object to cold object
conduction => 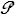 = k A dT/dx ; look up Thermal Conductivity k , a material property , in a table . . . k is NOT kB ! = k A dT/dx ; look up Thermal Conductivity k , a material property , in a table . . . k is NOT kB !
convection => 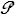 goes as T , or T 3/2 , or T 2 , depending on type & geometry goes as T , or T 3/2 , or T 2 , depending on type & geometry
radiation => 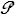 = σ ε A T 4 ; here , σ = 5.67E−8 [W/m² K 4] , and ε is surface emissivity = σ ε A T 4 ; here , σ = 5.67E−8 [W/m² K 4] , and ε is surface emissivity
Heat changes a system's Temperature (perceived random translational KE) => Q = "m" c ΔT , where c is the material's specific heat capacity ... see UNITS in your table !
or changes a system's state => Q = m Lf or m Lv . . . I.E. leaves as mass freezes, enters as mass vaporizes
hot objects are slightly bigger => ΔR / R = α ΔT ; look up α in a table , as a property of the material
Topic 11 Summary :
When an object or gas sample is allowed to expand, it does positive Work on its environment
. . . Force by the gas applied to its container is along the container's displacement
=> Wby gas = PΔV during any process .
If the piston which contains the gas has small mass , so that it always has small KE ,
. . . and if the Force applied to the outside of the piston by the external world is variable
(caused by an external device, as if the piston rod connects to a crank shaft) i.e, not constant pressure
. . . then the Force by the gas (outward) ≈ − Force by outside world (inward)
and the Work done by the gas is approximately the Work done on the external device .
In actual practice, the Work done by the gas is always more than the Work done on the device.
. . . Engineering Techology strives to make these two Forces as equal as possible
but they cannot be equal except in the limit accel = 0 ... which implies infinite time to expand
. . . faster expansions always mean that a smaller fraction of the gas Work is transferred to the device.
One way to describe of the effectiveness of a device design is the ratio : measured "Work from device" / "Work by gas"
. . . but by considering Energy Conservation , we'll find a more generally-useful definition .
If everything were always the same Temperature, no Thermal Energy would ever flow .
. . . the gas in the cylinder achieves a high Pressure (relative to the outside) because its Temperature changed .
To do another expansion , later , the gas in the cylinder needs to become low Temperature beforehand
. . . we can either dump that hot gas directly into the environment (say, through a valve)
and get new fresh cool air from outside (through another valve) - which seems wastful ...
. . . or we can let Heat flow out of it , through the cylinder walls and bottom
by putting the cylinder in contact with cool outside material - which sounds slow .
. . . Both methods transfer the same amount of Energy to the cold environment
this "rejected" Energy is Energy that can not do Mechanical Work on the external device :
=> Wby gas, max = Qfrom hot − Qto cold .
The traditional way to describe how "good" a device is, is by Energy efficiency :
=> e = ΔEdesired / ΔEpaid_for .
Devices are named according to the Energy change whose outcome is desired :
. . . Engine : we desire Mechanical Energy out from the device (e.g, ΔKE or &DeltaPE)
in a Heat Engine , we pay for the Q coming from from the hot material (e.g, fuel burned)
{in specialty Cold Engines , we pay for the Q going into the cold material (e.g, evaporating liquid He or liq.N2) }
. . . Refrigerator : we desire Thermal Energy out from the cold reservoir (the inside of the refrigerator)
we pay for Mechanical Energy (to compress+expand a working fluid) to extract this Qcold
(working fluid is very hot after compression , so IE flows from it into the warm kitchen ;
(... then fluid is very cold after expansion so absorbs Q from freezer)
{exotic Heat refrigerators combine a Heat engine with a mechanical refrigerator, as in "Back To the Future 1865" }
. . . Heater : we desire Thermal Energy into the hot reservoir (e.g, a house in Winter)
with Electric heaters , we pay for Electrical Energy ; almost all goes to the hot reservoir (transformed in the house)
for Chemical heaters , we pay for Chemical PE (H2, wood, methane, kerosene, coal) , seldom for the oxidizer
. . . some E escapes to the cold reservoir "up the chimney" , after combustion
with a Mechanical "Pump" heater (Heat Pump) , we pay for Mechanical Energy (or Electric Energy to run a motor)
... which essentially refrigerates the cold reservoir (outside air, or ground-loop)
Four "special" processes in a closed system typically occur (many processes are approximately one of these) :
constant Volume : "IsoChoric" or "isoVolumetric" process . . . Work W = 0 , so it is simplest scenario (but uncommon) ;
. . . Q = ΔI.E. = (d/2)NkBΔT ... with d modes of motion (3, 5, 6...) ... all the Heat input stays inside, producing ΔT
and the Specific Heat Capacity Per Molecule is just cgas,const.Vol = (d/2) N kB(m/molecule) .
. . . an isochoric process is done in a pressure vessel , to keep T/P constant ... or else vaporized matter escapes !
constant Pressure : "IsoBaric" process . . . Work = PΔV , so
. . . Q = PΔV + (d/2)NkBΔT ≈ (d/2 + 1)PΔV .
that is, (m/molecule) cgas,const.P = N kB + (m/molecule) cgas,const.Vol , due to Work done during expansion .
. . . if your container is NOT sealed, then vapors can escape or condense => an "open system" , not an isobaric process.
it is unusual to have T/V constant in a closed container !
constant Temperature : "IsoThermal" process . . . PV = constant , so W = N kBT (ln{Vf/Vi)
. . . Work is the Area under the curved isothermal line on the PV diagram { from integrating P dV = (NkT/V)dV }
Q = W , because the Thermal Energy (molecular KE) depends only on Temperature (which doesn't change)
. . . slow process in a constant-Temp environment will be isothermal ; the Work it does is offset by the Heat done to it .
isotherms are "opposite" isochors in this sense: NO Heating stays in as ΔT - it all goes out as Work.
constant subsystem Energy : "ADiabatic" ("no heating") or "IsoEntropic" ("constant Entropy") process
. . . Q = 0 , so W = ΔI.E. = (d/2)PΔV .
W = ΔI.E. , so expansion causes the Temperature to decrease , with no Q input to offset it.
. . . poly-atomic molecules, holding E in more modes, change T less than monatomic molecules do , for the same P's
PV γ = constant1 ... here, γ = cP/cV = (d+2)/d : 5/3 for monatom, 7/5 for diatom, 8/6 for bent molecule
... or TV γ − 1 = constant2 = constant1 / N kB
so W = Δ(PV)/(γ−1) . . . with 1/(γ−1) = d/2 ~ 3/2 , 5/2 , 6/2 .
. . . if a process is fast (rapid) , or well-isolated (insulation or size) , it is approximately adiabatic ;
(1) rising air expands & cools as P decreases ... (2) piston compresses diesel fuel mix until high T ignites it.
. . . adiabats are "opposite" isochors in the sense of switching "mechanical" with "thermal"
. . . adiabats are "opposite" isotherms in the sense of switching internal with external Thermal Energy
A very special cycle is made from isothermal at Thot, then adiabatic , then isothermal at Tcold, then adiabatic to Thot .
. . . called a "Carnot" cycle (for N.L.Sadi Carnot, 1824), it is the most efficient cycle possible for a Heat Engine .
=> eH.E.max = W/Qhot = 1 − Qcold/Qhot = eCarnot = 1 − Tcold/Thot .
Instead of showing a process on a PV diagram, which has Mechanical Work as its Area,
we might graph different variables for the same process , which would display Thermal Heat as its Area .
An adiabatic process must be a vertical line on such a diagram , so the Area under it would be zero
. . . the Q analogy to the W=0 isochor on a PV graph ... whose Q all becomes ΔIE ...
for an adiabat , the Work done all becomes ΔIE ;
=> Temperature T , in Kelvin, is the thermal quantity which changes during an adiabatic process .
Since Temperature is the vertical axis of our new "Area=Heat" graph, the isotherms are horizontal lines ;
. . . they appear to be perpendicular to the adiabats, from the perspective of Heat .
What quantity, describing the condition of the gas, is constant during an adiabatic process?
{ the Heat Q describes the process, not the initial and final conditions }
. . . it must be an extensive quantity , since Temperature is intensive (analogous to Pressure) ;
its change during an isotherm , multiplied by Temperature , must be the Heat Q absorbed ;
Entropy S must accumulate like Internal Energy divided by Temperature :
=> ΔS = Q/T , so that T · ΔS = Q
On a T vs. S graph , the Area under a curve is the Heat required ;
. . . system absorbs positive Heat if its final Entropy is larger than its initial Entropy . . .
one may think of S as accompanying Q thru the system boundary
{ similar to how momentum is always exchanged while Mechanical Work is transfered )
. . . concentrations have low S , and tend to dissipate
concentrated Energy => high Temperature , which gets conducted away ;
concentrated molecules => high Pressure , which pushes on the boundary ;
concentrations of any particular kind of atoms (say, ammonia) tend to diffuse away
Unlike other variables which describe a state or condition of objects or sets of objects (systems),
{ some are conserved, so the total is always the same }
{ some change as a conserved quantity transfers , so can increase or decrease }
. . . Total Entropy is either almost constant (for an infinitely slow reversible process) , or ΣS increases .
. . . the 2nd Law of Thermodynamics can be stated as "Total Entropy of an isolated system never decreases".
Entropy describes how much random chance the atoms have ... the number of ways that they could be arranged ... call Ω
=> S = kB ln(Ω) . . . S has units of [J/K] (like Boltzmann's kB !)
|