College Physics 2 (203) - Topic Ten Summary Notes
Science 159 (below 3rd Ave ramp) foltzc@marshall.edu don't phone - stop in!
Topic 9 (Matter Waves & Electrons In Atoms)
Readings for Topic 9 (from Knight Jones Field) : Ch.28 + Ch.29
|  |
plan for Topic 10 Quiz to be Tue.Apr.16 - new equations are described below,
but you may also use this full page of 203 eq'ns from now on
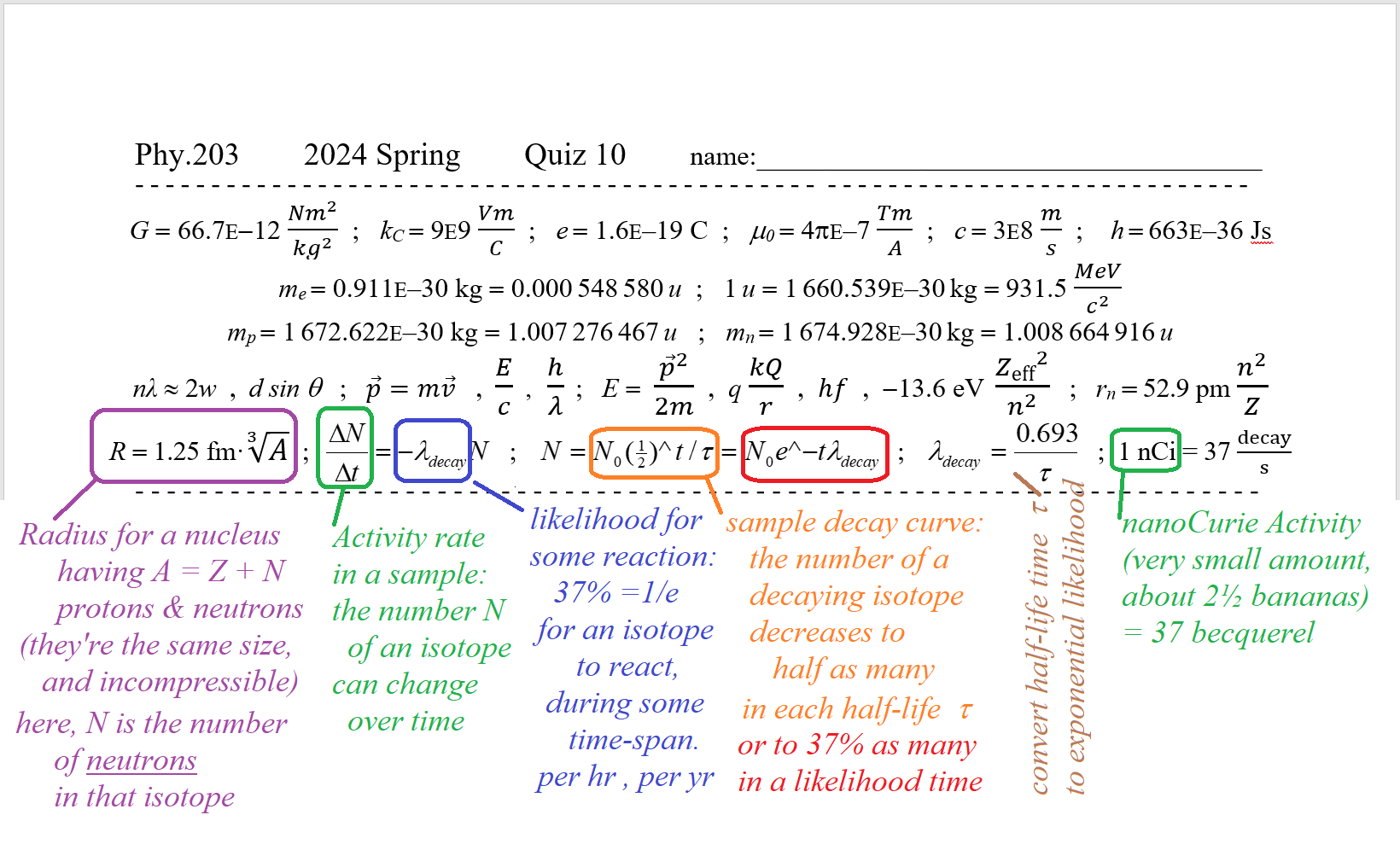
plan for Unit 2 mini-Exam (40 point) to be thRs.Apr.18
the Phy.203 Common Exam will be Sat.Apr.20 in Sci.277 at noon
Idea #20: atomic Nucleus made of Z protons and N neutrons ; Z + N = A - - - - - -
. . . . proton mass = 1.007 276 466 8 [u = "atomic mass units"] . . . 1 u = 1660.539 066 E−30 kg ; u c² = 931.494 026 MeV
+ electron mass = 0.000 548 579 9 [u]
hydrogen atom m = 1.007 825 032 1 [u] <= mproton + melectron − ionization Energy / c²
neutron mass = 1.008 664 915 9 [u]
electrons cannot fit in the nucleus; if a ½ wavelength is confined to a few fm (e.g, 83Li diameter = 5.0E−15 m ),
their p= h/λ= 1.35E-19 kgm/s, an electron would have KE = p²/2me ≈ 10 nJ (=62,000 MeV => effective mass m = E/c² ≈ 111.E−27 kg) ... each!
. . . the more massive neutron has similar wavelength and momentum, but much less KE (≈ 1/1840).
nomenclature by example: 136C½
Charge Number Z : the number of elementary charges (protons) in the nucleus or other object
. . . the element's NAME is determined by its charge number ... they are always integers
written as a SUB script to the left of each object's Chemical symbol (see the 6)
=> the total charge before any reaction MUST be the same as the total charge afterward! (charge is conserved)
Mass Number A : the number of "heavy things" ("baryons" ~ protons + neutrons) in each object
written as a SUPER script to the left of the symbol ... they are always integers (see the 13)
=> the total mass number after a reaction, IS the same as it was before the reaction => this integer "mass number" is conserved
. . . notice that A = Z + N, so the number of neutrons is "hidden" ... N = A − Z
the example's N = 13 − 6 = 7 neutrons in this "heavy carbon" ... 1 more than typical (6 in carbon-12)
... this is extremely unusual, being a stable isotope with an odd number of neutrons ...
only 1% of Earth's carbon is Carbon-13
The number of fermions ... things with "Spin Angular Momentum" s = ½ (h/2π) ... (see the ½)
. . . must be even (or odd) after the reaction, if it was even (or odd) beforehand . . . number of fermions is conserved (↑ spin + ↓ spin = 0)
photons have spin 1 (h/2π) , so are bosons, not fermions ... boson number is not conserved .
Nuclear Structure : Hard-sphere model - - - - -
neutron radius ≈ proton radius = 1R ≈ 1.25E−15 [m] = 1.25 [femto-meter]
. . . more nucleons => bigger radius : AR³ = A 1R³ => essentially incompressible volume
so "Copper-64" = 6429Cu has nuclear radius = 3√(64) · 1.25 [fm] = 4 · 1.25 fm = 5.0 fm
... its diameter is twice that of the A=8 Lithium nucleus mentioned above (rLi-8 = 2.5 fm)
. . . a nucleus would need to have A = 512 to have 10 fm radius − there aren't any nuclei that big
the biggest stable nucleus is 23892U ... (find out how to take a cube root on your calculator − its radius is 7.75 fm)
Strong Nuclear Force : every baryon (proton,neutron) attracts every other nearby baryon => negative Strong Potential Energy
. . . but strength decreases very steeply with distance , as a negative exponential besides the 1/r² spread ...F ≈ kS exp(−r/R)/r²
this R is a bit over a fm, but is distinct from R1 from the nuclear radius formula (above).
. . . so a nucleon obtains U = −2 MeV strong PE from each closest neighbor ... a nucleon might have as many as 12 of these that it touches ... not much from any of the others
v (−0.3 MeV from its next-neighbors, −.0008 MeV for 3rd neighbors)...
Electric Force : protons repel protons => positive Electric Potential Energy Σ q V , both +
. . . weaker than the Strong Force, but fairly long-range : V ≈ Σ kC (Z-1)e / r ... only 0.6 MeV from each neighbor proton, and 0.3 MeV from 2nd neighbors, but .2 MeV from 3rd , .15 MeV from 4th ...
this ultimately limits how many protons can be in a nucleus ... there are 9× as many 3rd neighbors as nearest neighbors
. . . the neutrons settle in among the protons, keeping them farther apart fom each other (except each proton will magnetically pair with one other)
Magnetic Force : nucleons pair up magnetically => negative Magnetic Potential Energy for each pair
. . . weaker than the other nuclear Energies , and very short-range (dipole Force strength applied to another dipole ~ 1/r4)
−0.35 MeV if they pair up correctly ( +0.35 MeV if they are oriented wrong)
( internal Gravitational Energies are only ½ G(A u)²/ r *1.6E-19 C/e ≈ 10^−65 eV , for Cu-64 , so totally ignoreable still )
empirically, nuclei seem to favor having even Z and even N ... about 10× as much as the small magnetic energy indicates
. . . that is due to Pauli exclusion, allowing 2 protons in their lowest available Energy level, or 2 neutrons in their lowest available Eenrgy level
if a nucleus has one odd proton and one odd neutron, the higher-level one will be able to lower its energy by changing to match the other nucleon.
they also seem to prefer having the same number of neutrons as protons, especially in the lighter nuclei ;
. . . the energy cost of being unequal seems to be about 27[MeV] |N−Z|/A ,
Application : any nucleus can eventually decay if its PE starts higher than it will be after the decay - - - - -
5 things a nucleus can do naturally to lower its Potential Energy
1: emit an alpha particle α = 42He ... just the nucleus (but it will eventually collect 2 electrons to become a neutral atom)
2: emit a beta particle β− = 0−1e ... simply an electron , just like the ones that surround most nuclei
3: emit a gamma ray γ = 00γ ... a photon , but usually 50× more energetic than an X-ray (1 MeV ≈ 50× 20 keV)
4: emit a positron particle β+ = 0+1e ... an anti-electron has the same mass & spin as as a regular electron , but has positive charge
5: capture an atomic electron ... a proton can neutralize its charge this way , becoming a neutron , if it can also emit (or capture) a neutrino (see details below)
Example 1 : Radon emits α ... the most stable Rn-222 has half-life 3.8235 days (<= looked up in data table)
. . . so its likelihood to decay λmean = 0.693/3.8235 d = 18.2% per day = 2.1E-6 per second
22286Rn0 → 42He0 + 21884Po0 + KE
step 1) charge conservation: the nucleus started with 86e ... and emitted 2e so there must be 84e left in its "daughter" ...
step 2) mass number: there were 222 heavy things, but 4 were emitted, so there must be 218 left in the decay product
step 3) angular momentum conservation: started with 0 spin+orbit ... the alpha has 0 spin and the Po daughter has 0 spin
... so the α and Po must come away with opposite orbital angular momentum L (or none)
. . . mean lifetime Tmean = half-life τ / 0.693 (⇐ ln[2]) = 5.5 days ≈ 475 kilo-seconds ... longer than a half-life
The initial radon nucleus has 3 aspects that make it unstable :
. . . 1) it is simply too big ... the short-range attraction from the strong Force holds together little bunches of nucleons (especially 4 , or 12 , or 28 , or 40 )
... huge globs with more than 200 can be shaken apart by their own momentum ... (they must have momentum, or their wavelengths would be ∞ ! )
. . . 2) it has way more neutrons (N = 222 − 84 = 138) than protons (86)
the bottom neutrons are in much deeper PE (more negative) than the bottom protons (they have negative strong PE , but lack the positive electric PE)
but the extra neutrons stack up so the top neutrons are in roughly the same total PE ("Energy levels") as the top protons
(sort of like electrons in atom energy levels ... the top ones are just barely trapped)
The nucleus that is most stable has protons and neutrons with the lowest (most deeply negative) Energy .
. . . this is called "binding Energy" per nucleon ... maximum is around 5626Fe ... 6028Ni , 6430Zn , 5224Cr are close
... notice that all the nuclei that are most tightly bound are even protons & even neutrons
. . . you can think of individual free protons and neutrons being assembled into a nucleus :
as they fall into the (strong negative) PE well, they radiate away (as γ-ray photons) their excess Energy , and become trapped .
=> the Energy that they have lost while forming the nucleus is the negative PE that they reside at while in there;
we would need to give them that much Energy, to "unbind" them and set them free again ... so it is called their Binding Energy .
the amount of Energy radiated by each new constituent (as the nucleus is being assembled) is typically 5 to 8 MeV or so
... 8 MeV is 8 E6 * e [Volt] = 1.28 pico-Joule ... for each nucleon! From E = m c² , the mass effectively radiated away is
roughly equivalent to m = E/c² = 14 E-30 kg each!
... each nucleon is only about 1673 E-30 kg , so the lost Energy shows up as a "mass defect" in the actual nucleus
(or when you measure the mass of the atom, which is a lot easier to actually do in the lab).
86 proton masses + 136 neutron masses + 86 electron masses = 86 (1 672.622 E-30 kg) + 136 (1 674.927 E-30 kg) + 86 (0.910938 E-30 kg) = 371 713.910 E-30 kg
. . . but the radon-222 atom actually has mass 368 668.835 E-30 kg . . . so its "binding mass-Energy" is Δm = 3045 E-30 kg ... Δm c² (/e) = 1712.8 MeV
that means its PE = −1712.8 MeV ... but dividing it among the 222 nucleons => − 7.715 MeV/nucleon ... find it on Kartunnen's graph !
. . . the stable nuclei about that size have binding Energies roughly 7.8 MeV/nucleon ... that is, 20 MeV (total) deeper .
the decay product masses don't add up , either ... this time I'll use atomic mass units (1 u = 1660.539 E-30 kg) :
helium-4 mass + polonium-218 mass = 4.002 602 u + 218.008 973 u = 222.011 575 , rather than the radon's 222.017 571 u
. . . the excess mass (0.005 996 u) in the parent radon , becomes KE in the products : (.005 996 u)*(1660E-30 kg/u) * c² (/e) => 5.6 MeV
on this very small size scale, you can think of "mass" as being "coagulated Energy" that is well-localized
. . . 3) it has too many protons, too close together ... the protons cause (positive) Electric Potential with long range (1/r)
the (negative, attracting) potential due to the strong Force goes to zero very nearby (e−r/r )!
=> the ratio N/Z needs to become larger than "1" for nuclei with a lot of protons Z .
. . . it makes most sense to treat proton number Z as the "independant" variable, with all of the various neutron numbers N vertically, as on the first chart
(rt-click to enlarge) ... but most isotope charts arrange Z ↑ and N→ , as chart 2 below . . .
. . . here is a link to Brookhaven National Lab's Interactive Chart ... very interesting, don't spend too much time browsing in it!
Half-life and Mean Life ; Activity and Decay Likelihood - - - - -
if you had a million Radon atoms you would expect 2 to decay each second ... ΔN / Δt = − 2/sec
. . . after 3 days (quarter million seconds) you would only have half million Radons left, so only 1 would decay each second
the negative simply means that the number of Radons decreases ... as the number of Poloniums (and Heliums) increases .
Mean Lifetime Tmean = 1/λ . . . THIS λ is likelihood to decay in some time span [/sec, /min, /hr, /yr].
any 2011Na is 65% likely to decay in 1 second . . . (by atomic electron capture, becoming 2010Ne + 00ν )
. . . that is, 2 out of 3 will capture an electron during the first second ... more than half of them.
. . . half of them do it in less time than that 1 sec : the half-life is shorter than the expected life.
half life = τ½ = Tmean· 0.693 . . .
. . . with a large sample N0 of items at time t = 0 ,
=> ΔN = − λ N0 will become something else during the likelihood time .
a high decay likelihood clearly means that lots of them will decay ... with λ ≥ ½ 1/s, has the majority not surviving the first second
. . . but each surviving radon has the same likelihood to decay during the next second , as they did during the first second. no memory that they are old
. . . since there are fewer remaining after that , the expected number to decay will also be less .
the number remaining (that haven't done it yet) decreases exponentially :
=> − ΔN/Δt = Decay Rate = N0 e^(−λ t) . . . this e ≈ 2.71828... is base of natural log)
. . . half-life τ½ is how long it takes for half a sample to be decay , so half is left ; (rather than 1/2.718 = 36.8 % to remain)
τ½ = (0.693147...)/λ . . . is shorter than the mean lifetime ;
=> decay rate ~ R = − ΔN/Δt = N0 (½)^(− t/τ) . . . the exponent is just "how many half-lives has it survived?"
a sample's decay rate is also called its radioactive Activity .
Because the number of undecayed parents decreases (exponentially) with time,
. . . 1) the mass of those undecayed parents also decreases exponentially with time: m = mo e−λ t
. . . 2) the activity of those undecayed parents decreases exponentially : A = Ao e−λ t
. . . 3) the rate of Energy release (Power) from them decreases exponentially : P = Po e−λ t
Example 1 : neutron decays into proton and electron . . . mean life-time 881 [s]
10n → 11p + 0−1β + 00ν
. . . the beta β− is an electron ... needed to conserve charge .
the neutrino ν , predicted by Pauli, evidenced by Cowan ... needed to conserve spin ;
. . . this is really an anti-neutrino , with negative fermion number , so the total number of fermions are strictly conserved
total momentum (3-d vector!) is also conserved in every reaction (relativistic kinematics often required)
in the neutron's c.o.m. frame, the decay products must be traveling in "opposite" directions . . . the 3 p vectors add to zero .
. . . if the neutron decays from rest, all 3 momentums have similar magnitude (but different directions) ... the proton's p = mv ...
the proton has 2000× as much mass as the electron, so the electron is going nearly 2000× as fast as the proton
... since KE ≈ ½p·v , the electron has nearly 2000× the KE as the proton ... and the neutrino carries even more than the electron!
. . . total Energy is also conserved , but only if you include "mass Energy"
mass: 939.565 [MeV/c²] → 938.272 [MeV/c²] + 0.511 [MeV/c²] + "0"
so KE: 0 [MeV] → 0.782 [MeV] KE . . . more KE ~ ½p·v in lower-mass pieces !
Half-life of a free neutron is 881 sec × ln2 = 881 s × 0.693 = 610 seconds ... about 10 minutes , so after an hour (=6 half-lives) only 1.6 % =(½)^6 will survive .
Example 2 : proton fuses with proton , making deuterium . . . initial (Threshold) Kinetic Energy is required !
11p+½ + 11p−½ + KE → 21H0 + 0+1β+½ + 00ν−½ + 00γ±1 .
. . . initial KE is needed so that the 2 protons can get very close together (2E−15[m] = 2 [femto-meter]) , which requires high PE :
PE = qV = q (kQ/r) ~ 0.72 [MeV] , to get +e to be 2[fm] from the other +e .
. . . anti-electron +β+ , fondly called a "positron" , has mass identical to an electron's , but has positive electric charge .
we've found antiparticles for protons and neutrons , and almost all the mesons , etc.
when positron and electron collide , all that is emitted are 2 gamma rays (opposite directions, opposite angular momentums)
Danger ! Energetic Projectiles are Emitted - - -
nasty UV light causes sunburn because the photons Energy (~5 eV) can break a dozen chemical bonds (say, in biological molecules)
Nuclear radiation typically carries 1-5 Million eV , so each projectile can ionize 100,000 atoms
alpha penetrate about 5 μm per MeV, so a sheet of paper (40 μm) can stop an 8 MeV α
they actually lose Energy less readily as they become slower;
. . . a 10 keV α penetrates 25 μm per MeV ... that is only 0.25 μm.
. . . concentrated Energy loss is good, if it happens in your shielding material,
and is not too bad if it is in your skin (those outer layers are dead already)
but it is bad if it occurs inside you ... say, via radon decay in your lungs.
beta lose only about .1 or .2 MeV per mm , so a 1 MeV electron penetrates about 10 mm
. . . until they lose momentum by this gradual Energy loss
a 10 keV electron loses Energy much more readily to the material it goes thru,
. . . because their wavelength becomes much longer, and their wavefront much wider
about 2.0 MeV per mm, so only goes 0.005 mm = 5 μm farther as it stops.
so beta radiation will ignore your skin and outer layers of fat and muscle,
. . . to deposit most of its Energy deeper inside some vital organ.
. . . All along the beta's path, it has ionized lots of atoms that it passes
10,000 ions per mm at first, means 1 ion every 100 nm ; only hits ¼ %
200,000 per mm at the end, means 1 ion every 5 nm ; some 5 % along the track get ionized
gamma are 0.4 % likely to be absorbed in each meter of air that they go thru
(compare: 50 keV x-rays are 4% likely per meter of air)
. . . so half of the gammas will make it thru (0.693)/0.004(1/m) = 178 meters of air ... ¼ of them thru 356 m , etc.
6 or 8 of those 178m thicknesses (1400m) gets rid of almost all the original gammas.
. . . density of water (we are essentially water) is 770× the density of air, so
gammas are 0.3% likely to be absorbed in 1 mm of water (or tissue)
gammas that enter a person will impart about half their Energy to that person's first 200mm (8") thickness
. . . with another ¼ of their energy being deposited in the next 8" (exit-half side of the body).
. . . a typical gamma will first ricochet ("Compton scatter") from an atomic electron :
momentum conservation means that the electron recoils when it is hit, knocked out of its atom.
. . . the gamma has less Energy after the richochet (so longer wavelength, still speed c)
the ejected electron might have enough KE to ionize several dozen atoms along its track
. . . the ion which lost that electron probably emits a UV cascade as the vacancy is filled in
. . . the gamma ricochets several times ; farther apart at first, when it has its highest Energy (smallest wavelength)
closer together as it loses Energy to these electrons (a larger gamma hits more of them).
. . . eventually the gamma gets absorbed by an atom as it knocks out a deep atomic electron
that atom will emit an x-ray, as a high-n electron fills the vacant hole ... UV cascade.
positrons (the anti-electron) find an electron to annihilate with almost immediately
the Energy in the positron (KE + m c²) plus the Energy in the electron (m c²)
is shared among 2 gammas ... each is at least 0.511 MeV (in the center-of-momentum reference)
. . . these gammas go outward from the annihilation point , acting like gammas (see above)
. . . the atom that had an electron annihilated fills in its vacancy via UV cascade.
Deeper than the Nucleus : the Standard Model of matter (leptons , quarks , & gluons) - - -
Each Baryon seems to be made of 3 quarks ;
. . . quarks seem to have Z = ±1/3 e, ±2/3 e
proton is made of 2 "up" quarks + 1 "down" quark
. . . Z = 2 (+2/3) + 1 (−1/3) = 4/3 − 1/3 = +3/3 = +1 . . . e's of course.
. . . A = 2 (1/3) + 1 (1/3) = 1 . . . each quark is 1/3 of a baryon
. . . s = (1/2) − (1/2) + (1/2) = 1/2 . . . 3 fermions make a fermion
neutron is made of 2 "down" quarks + 1 "up" quark
. . . Z = 2 (−1/3) + 1 (+2/3) = 0/3 = 0 . . . of course.
Each meson seems to be made of 2 quarks ;
pion . . . π+ = "up" + "anti-down" . . . π− = "anti-up" + "down"
. . . verify that their charge adds up correctly (the antiparticle has opposite charge)
. . . s = (1/2) − (1/2) = 0 . . . 2 fermions make a boson
|